Separating Mixtures: Using distillation to concentrate ethanol Virtual Lab
Rescue a town from a fuel crisis by using your knowledge about matter and phase changes principles and performing ethanol distillation.
Try our lab safety simulation
Discover one of our 200+ learning simulations today
- Description
- Features
About the Separating Mixtures: Using distillation to concentrate ethanol Virtual Lab
This short, targeted simulation is adapted from the full-length “Matter and Phase Changes: Distil ethanol” simulation.
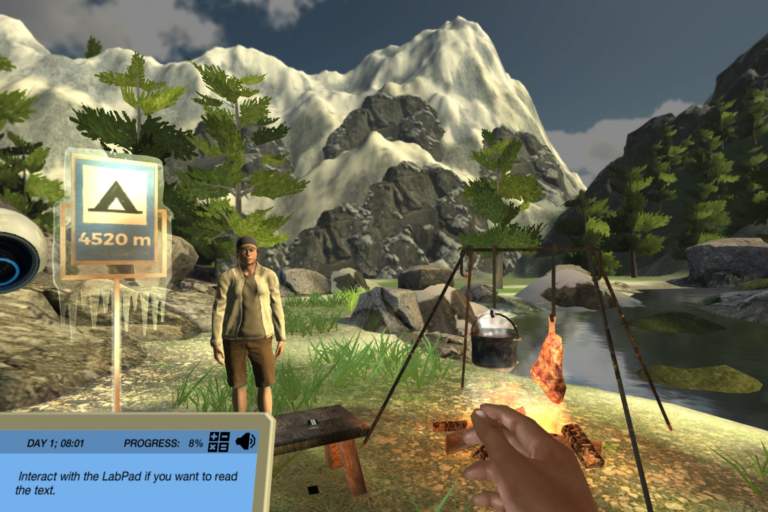
Get ready to encounter how great the distillation technique is! In this simulation, you will help a town concentrate ethanol in order to support them during their fuel shortage crisis. Are you ready to learn how to separate a homogeneous mixture of liquids?
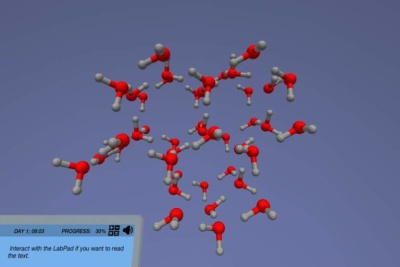
Explore the difference between physical and chemical change
在分离混合仿真,将我et Jo who has a limited fuel supply and therefore wants to produce bioethanol. In order to help her, you must first know the difference between the physical and chemical change of a substance in order to find the right technique.
Generate heating curves
Once you’ve mastered the basics, you are ready to learn about additional aspects of phase changes and be introduced to heat curves. You will learn how to interpret heating curves to identify important information of the boiling point of ethanol and water at atmospheric pressure. By doing so, you will be able to decode the meaning of each line of the heating curves and understand how this information is useful to your experiment. You will also be introduced to phase diagrams and understand how other physical properties can be predicted through this data.
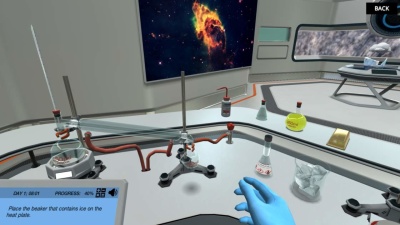
Perform distillation
Once you have understood why distillation is the right technique, the next step is to perform it. At the end of the simulation, you will put your newly acquired knowledge to the test! You are going to use the distillation technique to achieve ethanol with higher purity. Will you be able to produce high purity ethanol and report your findings to Jo?
Rescue a town from a fuel crisis by using your knowledge about matter and phase changes principles and performing ethanol distillation.
At the end of this simulation, you will be able to...
- Read a phase diagram and explain each region
- Interpret a heating curve of a given substance
- Explain the difference between a heating curve and a phase diagram
- Understand the basic steps of performing a distillation
- Distinguish between physical and chemical properties of matter and classify changes of matter as physical or chemical
Length: 16 Minutes
Accessibility mode: Available
Languages: English (United States)
Early Stage Bachelors Level
EHEA First Cycle
EHEA Short Cycle
FHEQ 5
FHEQ 6
FHEQ 4
US College Year 2
US College Year 1
Support for laboratory skills at all levels
HS-PS1-3, HS-PS1-4, HS-PS1-8, HS-PS1-5, PS1.A-E2
Chemistry 4.4 Intermolecular forces
Chemistry 3.1. Intermolecular Forces
Chemistry 3.3 Solids, Liquids, and Gases
Chemistry 4.4 Physical and Chemical Changes
Chemistry 6.5 Energy of Phase Changes