States of Matter Virtual Lab
Dive into water at the molecular level to learn about the behavior of the molecules in the three different states of matter: solid, liquid and gas. Learn about the bonding interactions between the molecules during different phase transitions.
Try our lab safety simulation
Discover one of our 200+ learning simulations today
- Description
- Features
About the States of Matter Virtual Lab
这个简短的,有针对性的模拟改编自the full-length “Matter and Phase Changes: Distil ethanol” simulation.
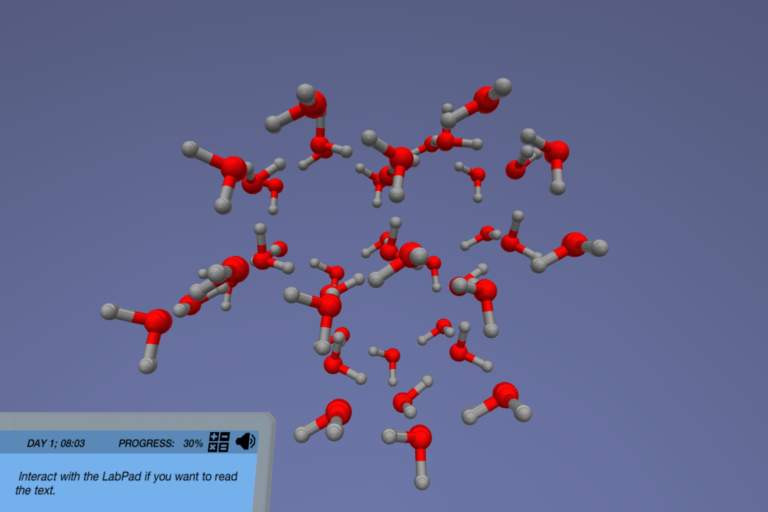
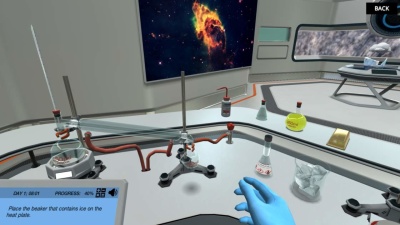
Get ready to encounter all three states of matter. In this simulation, you will manipulate the states of matter and inspect the numerous possible phase changes of a single substance. Will you be able to see that almost everything you interact with matters?
Identify the states of matter
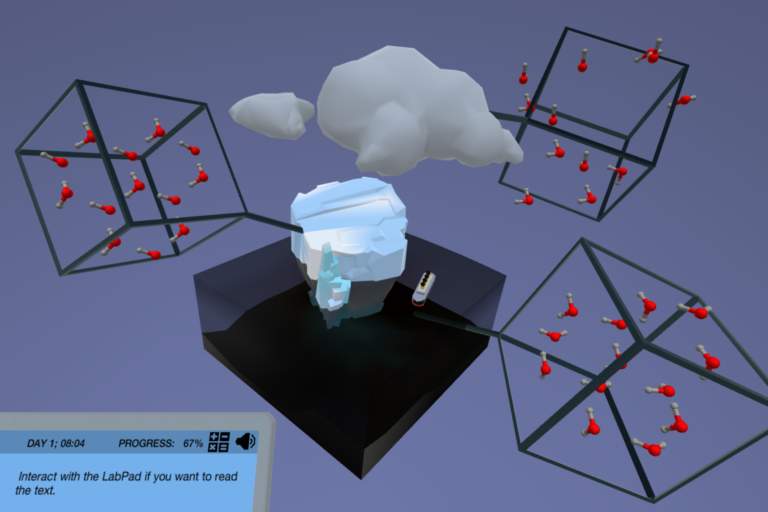
In this simulation, you will get the chance to explore Dr.One’s favorite movie scene using our high tech hologram! You will use it to explore physical representations of the three states of matter using water as our example. Can you guess what movie scene would be the perfect example to use?
Explore the intermolecular forces in matter
In the States of Matter simulation, you must first master the definition of matter and the interaction of the molecules at each phase. Only then will you be able to apply it to the process of phase changes. You will dive into water at the molecular level and examine the dynamics of intermolecular forces of the molecules during the phase changes. With the help of 3D animations, you will learn about the molecular configuration in three states of matter: solid, liquid, and gas.
Review your knowledge
With the help of the 3D animations, test your knowledge on the different phase transitions. Draw upon the knowledge that you had learnt previously about the behavior of a substance in each state of matter at the molecular level. Answer the quiz questions with the help of our insightful theory pages to better your learning on the whole topic.
Dive into water at the molecular level to learn about the behavior of the molecules in the three different states of matter: solid, liquid and gas. Learn about the bonding interactions between the molecules during different phase transitions.
At the end of this simulation, you will be able to...
- Explain solid, liquid, and gas states in terms of particle interaction and bonding energy
- Describe and explain the characteristics of a phase change
- Name the main phase changes: boiling, evaporation, freezing, melting, and sublimation
Length: 10 Minutes
Accessibility mode: Available
Languages: English (United States)
HS-PS1-3, HS-PS1-4, HS-PS1-6
Chemistry 4.4 Intermolecular forces
Chemistry 2.2 Intermolecular Force and Potential Energy
Chemistry 3.1. Intermolecular Forces
Chemistry 3.2 Properties of Solids
Chemistry 3.3 Solids, Liquids, and Gases
Chemistry 4.4 Physical and Chemical Changes
Chemistry 6.5 Energy of Phase Changes